Latest News & Features
Refine search
Americas
The healthcare company’s chief IP counsel is researching how law firms and patent attorneys are using AI tools to inform its own guidelines for external legal providers. 5 March 2026
Americas
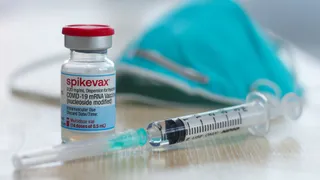
Moderna settles, agreeing to part with up to $2.25 billion to avoid a trial and secure a licence to continue using the platform underpinning its COVID-19 vaccine. 5 March 2026
Careers
As the firm expands its IP offering, “an unusually versatile lawyer” with chemical, biologics, and medical device spaces expertise joins the New York office. 4 March 2026
Careers
Launch forms part of the firm’s continued national growth strategy, boosting its presence in “a vibrant ecosystem of technology, life sciences, wireless communications and emerging growth companies”. 4 March 2026
Americas
With an increase in ‘hallucinated’ case law cited as evidence in courts globally, what can legal practitioners—as well as providers of AI tools and platforms—do to help safeguard against this? Sarah Speight finds out. 4 March 2026
Careers
A former Pinsent Masons partner has joined the firm’s London office, boosting the team’s capabilities in high-value life sciences arrangements. 3 March 2026
Europe
After an overwhelmingly positive Supreme Court ruling for companies working in AI, Rachel Free of CMS outlines the necessary practical steps for patent holders—including enforcement of the ‘black box’ tech, the ruling’s influence on the UPC, and how to manage related applications. 3 March 2026
Americas
Law firms, companies and individuals have been shortlisted across a range of categories, which this year include new awards for excellence in PTAB, trade secrets and medical device work. 2 March 2026
Americas
The companies have agreed to dismiss their long-running patent fight over tumour-informed liquid biopsy technology, closing a high-stakes chapter in the fast-growing MRD testing market—while leaving the door open to future claims. 27 February 2026
Americas
As the US Supreme Court prepares to hear arguments in the Hikma and Amarin dispute, the case has drawn a broad coalition of industry support—including the US government and a co-author of the Hatch-Waxman Act itself. 26 February 2026